By: Dr. Coni Horndli PhD.
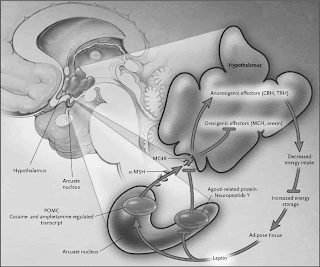
This recent study in Cell from Brad Lowell's group reveals novel circuits in the arcuate nucleus of the hypothalamus that selectively drive energy expenditure. The authors integrate genetic and
electrophysiological approaches with studies of metabolism and neuronal circuit
mapping to reveal a defined population of GABAergic neurons that regulate brown fat and energy expenditure. It remarkable to see such potent neural control over these aspects of physiology.
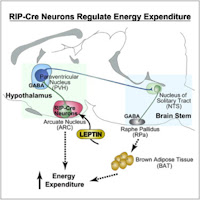
The authors use an elegant double-labeling method to reveal functional
neuronal connectivity between RIP-Cre ARC - PVH - NTS neurons. First, a
Cre-dependent virus, which expresses a channelrhodopsin-mCherry fusion protein
(AAV-Flex-ChR2(H134R)-mCherry) was stereotaxically injected in the ARC of RIP-Cre
mice. Second, retrograde green fluorescent beads were injected into the NTS of
the same mice. Electrophysiological recordings specifically showed light-evoked
IPSCs in bead+ PVH neurons.
In this study, a synthetic receptor was expressed in RIP-Cre mice and
activated by the pharmacological compound CNO. Neuronal activity in response to
CNO and later leptin were recorded in cultured brain slices. It would be
interesting to see whether the same neuronal responses can be elicited using an
in vivo approach.
In the ARC, only 30% of RIP-Cre neurons showed an excitatory response to
leptin, while 35% of ARC RIP-Cre neurons were inhibited. If and how ARC neurons
exhibiting these differential responses integrate into one circuit and or what
the role of leptin-inhibited ARC RIP-Cre neurons is in regulating energy
expenditure is not clear at this point. Also, I am wondering how, where and
when AgRP, which reside in the ARC, project to the PVH and are also GABAergic
but inhibit energy expenditure and food intake interplay in the RIP-Cre ARC -
PVH - NTS circuitry.
REFERENCE AND ABSTRACT:
GABAergic RIP-Cre Neurons in the Arcuate Nucleus Selectively
Regulate Energy Expenditure
Dong Kong, Qingchun Tong,
Chianping Ye, Shuichi Koda,
Patrick M. Fuller, Michael
J. Krashes, Linh Vong,
Russell S. Ray, David
P. Olson, and Bradford B. Lowell
SUMMARY
Neural
regulation of energy expenditure is incompletely understood. By genetically
disrupting GABAergic transmission in a cell-specific fashion, and by combining
this with selective pharmacogenetic activation and optogenetic mapping
techniques, we have uncovered an arcuate-based circuit that selectively drives
energy expenditure. Specifically, mice lacking synaptic GABA release from
RIP-Cre neurons have reduced energy expenditure, become obese and are extremely
sensitive to highfat diet-induced obesity, the latter due to defective diet
induced thermogenesis. Leptin’s ability to stimulate thermogenesis, but not to
reduce feeding, is markedly attenuated. Acute, selective activation of arcuate
GABAergic RIP-Cre neurons, which monosynaptically innervate PVH neurons
projecting to the NTS, rapidly stimulates brown fat and increases energy
expenditure but does not affect feeding. Importantly, this response is
dependent upon GABA release from RIP-Cre neurons. Thus, GABAergic RIP-Cre neurons
in the arcuate selectively drive energy expenditure, contribute to leptin’s
stimulatory effect on thermogenesis, and protect against diet-induced obesity.
|
|